

| Home Page | Overview | Site Map | Index | Appendix | Illustration | About | Contact | Update | FAQ |
| Period (MYA) | Environment | Process(es) | # of Minerals | Examples |
|---|---|---|---|---|
| 13600-Present | Since reionization | Supernovae | 0 | Heavy Elements |
| 13600-Present | Cool Envelope of Stars | Condensation | Dozen | Silicate Particles, Carbon Grains |
| 4540-4400 | Formation of Earth | Melting, Collisions | 200 | Olivine, Zircon |
| 4400-2000 | Black Earth | Melting, Weathering | 1500 | Beryl, Tourmaline |
| 2000-700 | Red Earth | Oxidation | 2500 | Rhodonite, Turquoise |
| 700-400 | White Earth | Glaciation Cycles (Re-distribution) |
2500 | Kaolinite, Ice |
| 400-Present | Green Earth | Bio-chemistry | 4400 | Aragonite, Calcite |
 |
 |
Rocks are aggregates of minerals - usually several, but sometimes only one or two. Minerals are either free, uncombined native elements (such as gold, silver, and copper), or elemental compounds (such as silicates - metallic elements combine with the Si-O tetrahedral radical). Gemstones are minerals suitable for use in |
Figure 09-06m Rocks, Minerals, and Gemstones [view large image] |
Figure 09-06n Gemstones Worldwide [view large image] |
jewelry after cutting and polishing (Figure 09-06m). They are rare, and therefore valuable, because their formation requires a highly unusual set of geological |
 |
 |
Since the Earth's crust composed mainly of Oxygen (46.6%) and Silicon (27.7%) for a total of 75%, the predominant compositions in minerals and thus in rocks are compounds such as quartz (SiO2), feldspars (XAl1-2Si3-2O8, where X can be either the elements Na, K, or Ca), and Mica (...Si3O10...) (see Figure 09-06o). There are three types of rocks according to the formation process (see Figure 09-06p, and Table 09-03). They are further sub-divided into different grain sizes and colors (light, medium, dark, not shown in the figure). |
Figure 09-06o Minerals in Rocks [view large image] |
Figure 09-06p Types of Rock |
| Type | Formation | Characteristic | Composition | Examples |
|---|---|---|---|---|
| Igneous | Solidified from molten magma either at the Earth's surface (extrusive) or underneath (intrusive). | The crystals can be very large (via slow cooling), and mostly have random distribution |  |
Basalt, Granite |
| Metamorphic | Created when existing rock is chemically or physically modified by intense heat or pressure, e.g., in collision of crustal plates | Have either wavy foliation (layer) or more random arrangement |  |
Gneiss, Schist |
| Sedimentary | Formed from erosion, transportation and subsequent deposition of pre-existing rocks or other kinds of sediments | May occur in layers, grains may be poorly held together |  |
Shale, Sandstone |
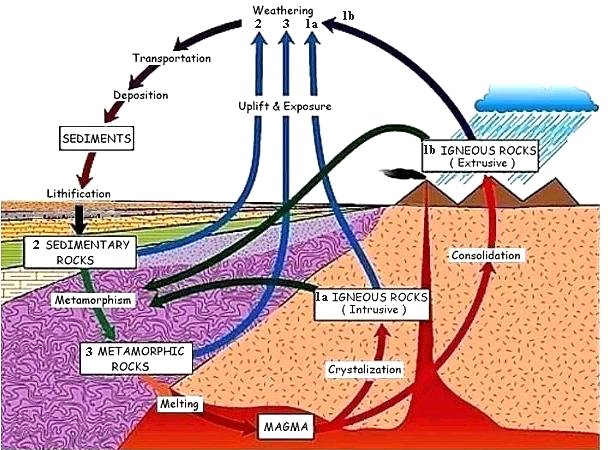 |
1a,b. Magma (molten rock) inside the Earth's crust rises through cracks and cools slowly underground forming igneous rocks composed of minerals with fairly large crystal sizes, these are known as intrusive igneous rocks. When the magma erupts onto the surface, as through a volcano, it is termed lava, the rapid rate of cooling makes the extrusive igneous rocks to form with medium to very small mineral crystals. 2. Once on the surface, the forces of erosion and weathering produce smaller particles (sands), which accumulate and compactify by pressure from upper layers to become sedimentary layers (rocks). 3. When sedimentary and igneous rocks are subjected to intense heat and pressure such as in the collision of the crustal plates, they turn into metamorphic rocks. Some of these are uplifted back to the surface by tectonic action. |
Figure 09-06q Rock Cycle [view large image] |
Further increases in temperature and pressure melt the rock deep under the crust into magma to complete the rock cycle. |
 |
1. Solution Precipitation - Near surface water becomes weak acid solution (with CO2 dissolved in it) in which many minerals are soluble. Gems will form as the water evaporates (Figure 09-06r (1)). Hot water from hydrothermal deep under are sometimes highly acidic or alkaline, making an even better solvent for more types of minerals. The slower rates of cooling and/or evaporation allow for larger crystals to form. Many of Precipitation - Near surface water becomes weak acid solution (with CO2 dissolved in it) in which many minerals are soluble. Gems will form as the water evaporates (Figure 09-06r (1)). Hot water from hydrothermal deep under are sometimes highly acidic or alkaline, making an even better solvent for more types of minerals. The slower rates of cooling and/or evaporation allow for larger crystals to form. Many of
|
Figure 09-06r Gem Formation [view large image] |
the world's highest quality specimens and metal ores have come form such environments (Figure 09-06r (2)). It may also appear as veins in the cracks (Figure 09-06r (3)). |
 Crystallization - This process is associated with the formation of igneous rocks. Large crystals can form in the intrusive type. The extrusive type generally not be expected to hold large crystals. Only rarely do larger gem crystals show up in a matrix of finer grained rock of this type.
Crystallization - This process is associated with the formation of igneous rocks. Large crystals can form in the intrusive type. The extrusive type generally not be expected to hold large crystals. Only rarely do larger gem crystals show up in a matrix of finer grained rock of this type. Condensation - Usually solid does not condense readily from the vapor phase. However, it does happen under special condition (such as frost on car windshields). If gases are trapped in bubbles within the lava, gems can crystallize upon cooling. Other pockets, which do not produce crystals originally, may later be invaded by surface water with mineral solution ultimately forming geodes or other similar formations.
Condensation - Usually solid does not condense readily from the vapor phase. However, it does happen under special condition (such as frost on car windshields). If gases are trapped in bubbles within the lava, gems can crystallize upon cooling. Other pockets, which do not produce crystals originally, may later be invaded by surface water with mineral solution ultimately forming geodes or other similar formations.| Class | Composition | # | Location | Examples |
|---|---|---|---|---|
| Silicate | Metallic elements + Si-O | > 500 | 95% of all rocks | Quartz SiO2, Garnet Mn3Al2(SO4)3 |
| Carbonate | Metallic elements + (CO3)-2 |  200 200 |
Marine and evaporitic settings | Calcite CaCO3, Dolomite CaMg(CO3)2 |
| Sulfate | Metallic elements + (SO4)-2 | Evaporitic settings, hydrothermal veins | Gypsum Ca(SO4) H2O, Barite Ba(SO4) H2O, Barite Ba(SO4) |
|
| Halide | Metallic elements + halogen |  100 100 |
Evaporitic settings | Halite NaCl, Fluorite CaF2 |
| Oxide | Metallic elements + oxygen | > 250 | Precipitates on Earth's surface | Ice H2O, Hematite Fe2O3 |
| Sulfide | Metallic/Semi-metallic elements + sulfur | > 300 | Metal ores | Pyrite FeS2, Chalcopyrite CuFeS2 |
| Phosphate | Metallic elements + (AO4)-3, where A can be P, As or V | Phosphate in teeth and bones | Pyroxmangite Pb5(PO4)3Cl, Bayldonite (Cu,Zn)3Pb(AsO4)2(OH)2  H2O H2O |
|
| Element | Chemical elements and alloys | Mines | Gold Au, Sulfur S, Silicides Fe3Si | |
| Organic | Carbon + hydrogen | Fossil fuels | Hydrocarbons CnH2n+2, CnH2n, CnHn |
| Property | Definition | Range | Examples |
|---|---|---|---|
| Structure | Defined by length of the crystal axes and the angles between them | Seven systems | Diamond (cubic), Quartz (trigonal) |
| Hardness | Mineral of higher hardness can scratch the surface of those with lower hardness | Mohs hardness in 10 scales | Calcite (3), Quartz (7) |
| Luster | Surface interaction with light | Seven kinds | Pyrite (metallic), Quartz (vitreous) |
| Color | Determined by impurity or internal structure | From red to violet including colorless | Ruby (red), Quartz (colorless) |
| Streak | True color in powdery form | Red to violet including white | Pyrite (dark green), Quartz (white) |
| Transparency | The amount of light passing through | Transparent, translucent, opaque | Pyrite (opaque), Quartz (transparent) |
| Cleavage | The way a mineral may split apart along various planes | Perfect, good, imperfect, none | Euclase (perfect), Quartz (none) |
| Fracture | The way a mineral may break contrary to natural cleavage planes | Conchoidal, sharp edges, fibrous, irregular | Euclase (conchoidal), Quartz (conchoidal) |
| Specific Gravity | Mass of the mineral relates to that of an equal volume of water | 1-2 (light), 2-4 (normal), >4 (heavy) |
Amber (1), Quartz (2.6) |