Mulitcellular Organisms
Cell Fate Manipulation
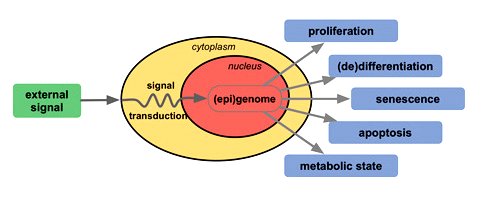
The fate of a cell is determined in the nucleus via an external signal. The presence or absence of certain growth factors in the medium is translated to a signal transduction, which cascades into changes of genome organization and associated activities. This in turn modifies the gene expression pattern and decides whether the call will differentiate, proliferate, go into senescence or apoptosis or adopt a specific metabolic state (Figure 10-14d). The path to different cell types is likened to a marble rolling downhill into one of several troughs representing fully differentiated cell types (Figure 10-14e, left). Nuclear transfer and reprogramming in embryo cloning showed that cells could be rolled back to the top of the hill (Fgiure 10-14e, right). It is the eggs that reset the nuclei of terminally differentiated cells back into the pluripotent state. Since then the quest for a similar feat without the egg's reprogramming machinery has been accomplished in spades. With the introduction of genes encoding a handful of transcription factors, multiple mouse cell types as well as human fibroblasts have been converted to an embryonic-like state. However, the process in creating specific cell types from there for regenerative medicine is rather inefficient with a long wait time. Turning a pluripotent cell into a neuron can take months in the lab, and some specialized human cell types can take much longer. Eventually report on July 2008 indicates that it is possible to transform mature cells directly into other cell types (the tunnelling in Figure 10-14e). Specifically, the technique turned digestive enzyme–producing pancreatic exocrine cells into insulin-secreting beta cells (in mice ). The latter are fairly rare to begin with and are in especially short supply in patients with diabetes, whereas the former comprise 95% of the cells of the pancreas. So, converting one into the other is roughly equivalent to turning copper into gold. The success seems to rely on the introduction of three transcription factor genes: Pdx1, Ngn3 and MafA. However the low efficiency of the process — mere hundredths to thousandths of a percent — and the fact that it takes close to a month to appear
 |
 |
suggests other actors besides transcription factors in setting cell fate. Some of these probable regulators are barely recognized as yet. For instance, the vast majority of all RNA transcripts in mammals may be non-coding such as the epigenome, suggesting that there's a lot more to learn about regulatory RNAs.
|
|
|
|
It was thought for a long time that cell fate is determined by signaling proteins and chemical gradients. It has been advocated for over thirty years that mechanical forces such as twisting, bending, and folding can induce different paths for the development of the stem cell. Such idea has not been received favorably until accumulation of more evidences in the 2000's.
 |
Researchers now know that almost all human cells test the mechanical properties of their microenvironment in the body, and use it to adjust their growth. This is determined by the extracellular matrix, a lattice of proteins and other molecules to which cells in solid tissues anchor themselves. The internal cytoskeleton includes a mesh of fibres made up of actin protein that lines the cell's membrane, plus tough actomyosin bundles in which actin combines with the protein myosin II. These two structures are joined together by integrins (a kind of protein), which span the cell membrane, gripping the actomyosin filaments inside |
Figure 10-14f Cell Membrane, Mechanical Structures  |
the cell and the extracellular matrix on the outside (Figure 10-14f). These structures together response to the push and pull with a lot of flexibility.
|
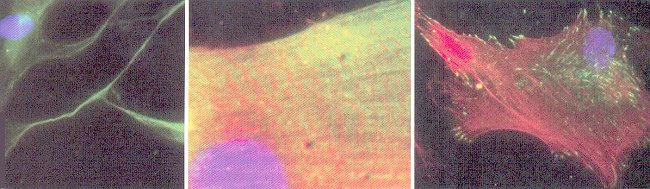 |
The effect is most obvious in astronauts' bones, which become thinner in the absence of gravity. Tissue stiffness is found to influence the growth or remission of cancer cells. The mechanical effect is most apparent in developing embryo where tissues twist, fold and writhe into the beginnings of adult tissues and organs. Figure 10-14g (left to right) shows embryonic stem cells grown on soft, medium
|
Figure 10-14g Mechanical Effects on Cell Fate  |
or rigid matrices start developing into neurons, muscle, and bone cells respectively.
|
6Epigenetics is the study of heritable changes in gene function that occur without a change in the DNA sequence. Epigenetic mechanisms includes histone modification, DNA methylation (replacing H with CH3), and RNA interference. DNA methylation is to add a methyl group to the DNA - frequently to the base cytosine when it is immediately followed by guanine. The methyl group can be sensed by proteins that turn gene expression on or off through regulating chromatin structure. Histone modification involves the chemical tags attached to the "tails" of the histones. There are more than twenty different tags, or certain combinations of them, that can either give rise to relaxed chromatin, which allows the assembly of transcription factors and transcription by RNA polymerase, or produce the opposite effect. If the DNA sequence of the genome is like the musical score in a song, then the epigenome is like the musical notations that show how the notes of the melody should be played. The sequence of the human genome is the same in all our cells, whereas the epigenome differs from tissue to tissue, and changes in response to the cell's environment. Their effects in gene inactivation and activation are increasingly understood to be very important in phenotype transmission and embryonic development.
.



