When energy (such as in a photon) is pumped into a chemical system, the energy partitions into thermal and electronic components. The thermal component makes the molecules move faster, and the electronic component increases the number of "high-energy" electronic states. Both energy components will foster molecular organization: the faster the molecules vibrate, rotate, and translate, and the more of them that are in electronic states above ground level, the higher is the probability that the molecules will interact and the more work can be done in organizing them. However, there is a limit to that. At very high energy levels all chemical bonds become inherently unstable, the molecular structures eventually fall to pieces. It draws the line to the energy input; it is impossible to make a macro- molecule in one run from scratch. It has to be made by supplying the required energy little by little. The aggregates (such as DNA or protein) are created by joining the units one at a time. This way each step of molecular synthesis could be driven by a separate and tolerable energy input.
Living organisms store photon energy in chemical form, and then trickle it down molecular chains to the individual molecular bonding sites. The energy flux that organizes all living matter on our planet is so channeled as to first pump CO2 and H in theThe flow starts with the capture of photons by certain molecules, such as the chlorophyll of plants and similar pigments of microorganisms according to the photosynthesis reaction:
6CO2 + 6H2O + energy
 C6H12O6 + 6O2
C6H12O6 + 6O2
Respiration runs in the reversed direction. While the energy input is carried by photons in photosynthesis, the energy output in respiration is distributed among a maximum of 6x(6 ATPs).
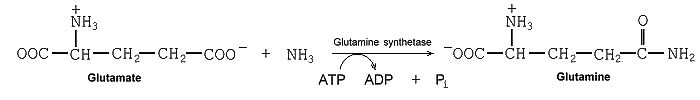
The photon energy is stored in the SP3 carbon hybridization -- about 6 quanta of red photons (~ 12 ev each) in one glucose molecules. From this reservoir, energy then flows along various pathways, nursing everything, all organization and all work. The chemical energy chains that nurse macromolecular organization commonly use ATP as their final link. Each package contains an energy of 7.3 kilocal per mole (~ 0.32 ev/ATP3). It is given off at the sites of amalgamation of the molecular building blocks -- one package for each site with spatial precision to where it is needed.(See "ATP synthase" for the production of ATP.)