| Home Page | Overview | Site Map | Index | Appendix | Illustration | About | Contact | Update | FAQ |
 |
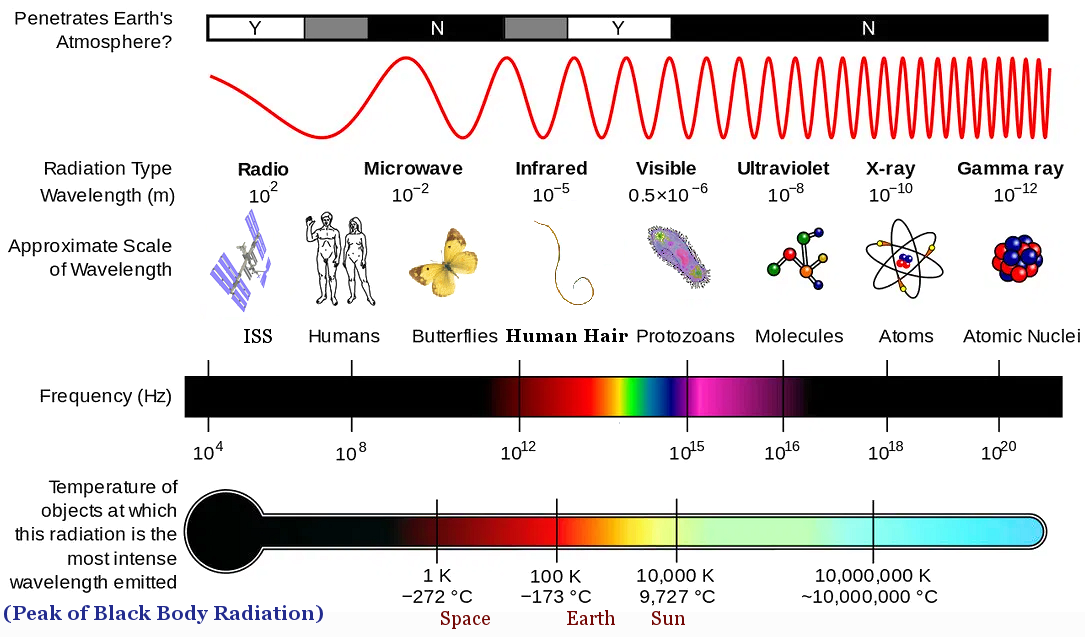
offer a consistent description. It is replaced by quantum theory, which describes the objects only in terms of probability, energy levels, and quantum numbers. A well-defined orbit similar to the path of a planet around the Sun becomes the probability of finding the object at a certain location in the microscopic world. Thus, all the illustrations related to these objects would be just a schematic diagram conveying some ideas, they should never be taken literally as the real thing (see objects smaller than 10-8 meter in Figure 12-01). Anyway, there is no such thing as probability, energy levels, and quantum numbers in nature; they are just abstractions invented by physicists to fit experimental data. |
Figure 12-01 Blackbody Spectrum [view large image] |
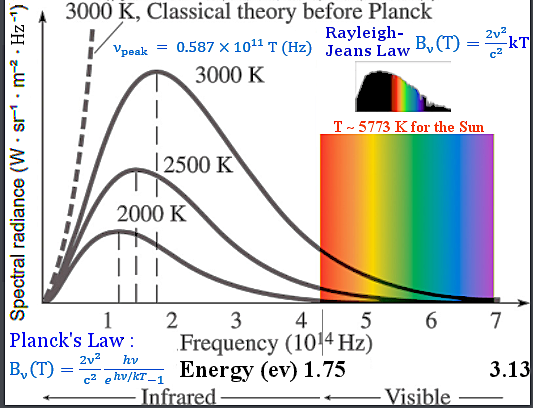 |
blackbody radiation. The classical theory of Rayleigh-Jeans failed to fit the observation of the radiation energy distribution from a blackbody at high frequency (Figure 12-02). In searching for a modification that would reduce the contribution of the high frequencies to the energy, Planck was led to make an assumption in 1899 : The energy of the radiation with frequency  is restricted to integral multiples
of a basic unit hv (a quantum), i.e., E is restricted to integral multiples
of a basic unit hv (a quantum), i.e., E = nh = nh where h = 6.625x10-27 erg-sec is the Planck constant and n is an integer. With this assumption, Planck obtained an exact fit to the observed distribution of radiation energy. According to classical theory, electromagnetic radiation is a wave phenomenon. The Planck's assumption endows a particle aspect to the same entity. Such wave-particle duality requires radical changes in the fundamental concepts of the properties of matter and energy. An introduction on the subject of "wave" can be found in the special topic on Wave, Sound, and Music, also see "Energy of Single Particle". where h = 6.625x10-27 erg-sec is the Planck constant and n is an integer. With this assumption, Planck obtained an exact fit to the observed distribution of radiation energy. According to classical theory, electromagnetic radiation is a wave phenomenon. The Planck's assumption endows a particle aspect to the same entity. Such wave-particle duality requires radical changes in the fundamental concepts of the properties of matter and energy. An introduction on the subject of "wave" can be found in the special topic on Wave, Sound, and Music, also see "Energy of Single Particle".
|
Figure 12-02 Blackbody Radiation [view large image] |
The mathematical formula for black body radiation (Planck's Law) is shown in Figure 12-02 in the form of spectral radiance (per unit frequency) B (T). Note that the added exponential factor bends the Rayleigh-Jeans curve at high frequency and they agrees at low frequency. (T). Note that the added exponential factor bends the Rayleigh-Jeans curve at high frequency and they agrees at low frequency. |
 |
 |
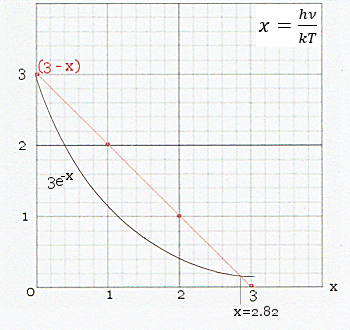
By defining x = h /kT, the Planck's Law becomes : /kT, the Planck's Law becomes :B  (T) = [2(kT)3/(h2c3)][x3/(ex - 1)]. (T) = [2(kT)3/(h2c3)][x3/(ex - 1)].For a given temperature T, the maximum radiation would occur at  peak determined by : peak determined by :dB  /dx = 0, /dx = 0,which yield (3 - x) = 3e-x. As shown in Figure 12-03, the solution of this algebraic formula is x = 2.82, or  peak = 0.587x1011 T (see Figure 12-02). peak = 0.587x1011 T (see Figure 12-02).
|
Figure 12-03 Peak Radiation [view large image] |
Figure 12-04 Radiation/wavelength [view large image] |
 peak = (0.29/T) cm.
peak = (0.29/T) cm. = -(d
= -(d /d
/d )B
)B = [2hc2/(
= [2hc2/( )5)][1/(ehc/
)5)][1/(ehc/ kT) - 1].
kT) - 1]. kT, the Planck's Law becomes :
kT, the Planck's Law becomes : (T) = [2(kT)5/(h4c3)][y5/(ey - 1)].
(T) = [2(kT)5/(h4c3)][y5/(ey - 1)]. peak determined by :
peak determined by : /dy = 0,
/dy = 0, peak = (0.29/T) cm.
peak = (0.29/T) cm.  peak cannot be derived from
peak cannot be derived from  peak as it would yield
(
peak as it would yield
( peak)' = 1011 T, which is about twice of what it should be. This is because
peak)' = 1011 T, which is about twice of what it should be. This is because  is inversely proportional to
is inversely proportional to  , i.e.,
, i.e.,  = c/
= c/ and d
and d = -(c/
= -(c/ )(d
)(d /
/ ) = -
) = - (d
(d /
/ ), i.e., it is not in a linear relationship.
), i.e., it is not in a linear relationship. B
B d
d = [2
= [2 (kT)4/(h3c3)]
(kT)4/(h3c3)] [x3/(ex - 1)]dx = [2
[x3/(ex - 1)]dx = [2 (kT)4/(h3c3)](
(kT)4/(h3c3)]( 4/15) =
4/15) =  T4,
T4, |
where  = (2 = (2 5k4)/(15h3c3) = 5.67x10-12 W/cm2-T4 is the Stefan–Boltzmann constant, and I(T) is known as the Stefan–Boltzmann Law (
Figure 12-05,a) formulated long time ago in 1879 by Austrian physicist Josef Stefan as a result of his experimental studies, the same law was derived again in 1884 by another Austrian physicist Ludwig Boltzmann from thermodynamic considerations. 5k4)/(15h3c3) = 5.67x10-12 W/cm2-T4 is the Stefan–Boltzmann constant, and I(T) is known as the Stefan–Boltzmann Law (
Figure 12-05,a) formulated long time ago in 1879 by Austrian physicist Josef Stefan as a result of his experimental studies, the same law was derived again in 1884 by another Austrian physicist Ludwig Boltzmann from thermodynamic considerations.Luminosity is the emitting power from the entire surface of the black body. In case of spherical black body such as the surface of star, L = 4  R2I(T) = 4 R2I(T) = 4 R2 R2 T4 (see Figure 12-05,b). T4 (see Figure 12-05,b).Such formula can be used to calculate un-measurable parameter from observable data. |
Figure 12-05 Blackbody Luminosity [view large image] |
For example, the radius of the Sun can be determined from its luminosity and temperature in the HR diagram by R = (L/4  T4)1/2 ~ 7x1010 cm (Figure 12-05,d). T4)1/2 ~ 7x1010 cm (Figure 12-05,d). |
 ) = 2/
) = 2/ 4, or per unit frequency n(
4, or per unit frequency n( ) = 2
) = 2 2/c3,
2/c3,
 |
where the factor of 2 is inserted for the 2 different directions of polarization, the difference of 4 is related to the inclusion of the solid angle of the cavity. is related to the inclusion of the solid angle of the cavity.By the "Equipartition Theorem", each mode of the standing wave has average energy of kT, thus we obtain the spectral radiance : B  (T) = (2c/ (T) = (2c/ 4)kT, or B 4)kT, or B (T) = (2 (T) = (2 2/c2)kT - the Rayleigh-Jeans Formula in spectral radiance. 2/c2)kT - the Rayleigh-Jeans Formula in spectral radiance.
|
Figure 12-06 |
 ) or n(
) or n( ); but the mode of standing (electromagnetic) wave
); but the mode of standing (electromagnetic) wave  n = n
n = n in classical theory is replaced by the quantum of energy (the photon as a particle) En = nh
in classical theory is replaced by the quantum of energy (the photon as a particle) En = nh , where h = 6.625x10-27 erg-sec is the Planck constant. The average energy is calculated by :
, where h = 6.625x10-27 erg-sec is the Planck constant. The average energy is calculated by :

 |
The formulation of Planck's Law is an example of phenomenological modeling, he just borrows the idea from the modes of standing wave and adds some modifications like the distribution of energy among the modes (the photons in the upgraded model). Another example is the Bohr atom (Figure 12-07), which transfers the standing wave pattern from straight line into a circle and assigns an energy to each level. Standing wave is a good analogy to quantum since the modes are in discrete amount of the fundamental mode. It is very useful in visualizing a stationary configuration. |
Figure 12-07 Bohr Hydrogen Atom [view large image] |
In mathematical terms, the relationship between standing wave and particle is established by the de-Broglie equation p = h/ and E = h and E = h , where p and E are the momentum and energy of the particle. Quantum theory is completely established by quantization of p and E, i.e., treating them as operators. , where p and E are the momentum and energy of the particle. Quantum theory is completely established by quantization of p and E, i.e., treating them as operators. |