 i
i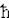 (d/dx) and E
(d/dx) and E  -i
-i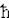 (d/dt). The followings are brief sketches to show the essence of the different methods.
(d/dt). The followings are brief sketches to show the essence of the different methods.- The nature of wave-particle duality was expressed mathematically by the de-Broglie relation (proposed in 1924) :
p = h/ ,
,
where p is the linear momentum of the particle and the wave-length of the wave.
the wave-length of the wave.
The duality was explained by the difference in measuring apparatus. For example, the double slit experiment would show the interference of the electromagnetic wave, while photons in the form of particle are involved in photoelectric effect. - It turns out that it is actually the probability wave (NOT the EM wave) causing the duality. According to this interpretation, the particle has a certain probability of being this or that following the related wave pattern. In addition, the "wave" may not be the traveling variety, it could be standing wave, or any kind of form. From this point of view, it can be shown that the momentum p should be expressed as an operator in coordinate space :

- Note that the commnutation relation is satisfied in this differentiation form for p and x, i.e., xp - px = [x,p] = i
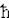 , which is related to whether p and x can be measured simultaneously
, which is related to whether p and x can be measured simultaneously
(= 0) or not ( 0), and the uncertainty principle
0), and the uncertainty principle  p
p x
x 
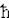 (see "Schwartz's Inequality"). This qunatization process also linearizes the classical equations with the dependent variable
(see "Schwartz's Inequality"). This qunatization process also linearizes the classical equations with the dependent variable  , which allows the superposition of different states creating all kinds of quantum weirdness such as being in 2 places at once (by applying quantum theory to macroscopic scale).
, which allows the superposition of different states creating all kinds of quantum weirdness such as being in 2 places at once (by applying quantum theory to macroscopic scale).
 |
|
Figure 12-05a Quantization |
See Figure 12-05a,a for an even shorter introduction. See original derivation in Chapter 9, "Quantum Theory" by D. Bohm, 1951. |
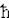 . The scheme is implemented by matrices as shown in Figure 12-05a,b. The replacement of ordinary number by matrix can be interpreted as turning a definite entity into a blurry one, and that has exactly been shown by its relationship with the uncertainty principle
. The scheme is implemented by matrices as shown in Figure 12-05a,b. The replacement of ordinary number by matrix can be interpreted as turning a definite entity into a blurry one, and that has exactly been shown by its relationship with the uncertainty principle  p
p x
x 
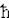 (see "Schwartz's Inequality").
(see "Schwartz's Inequality").The Hamiltonian formulation of classical mechanics treats the dynamic of the system in terms of energy (aka Hamiltonian H, see Figure 12-05a,c), instead of force (as F = ma in Newtonian mechanics). It involves a mathematical term called Poisson Bracket (PB). For the case of coordinate x and momentum p, the PB {xp - px} = 1. Quantization just replaces the PB with the commutation rule [xp - px] = i
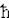 from which we obtain p
from which we obtain p  -i
-i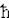 (d/dx), and all occurrences of p in the Hamiltonian H is substituted by such operator. See the wordy justification in 321 pages by P.A.M. Dirac in his 1926 thesis "The Principles of Quantum Mechanics".
(d/dx), and all occurrences of p in the Hamiltonian H is substituted by such operator. See the wordy justification in 321 pages by P.A.M. Dirac in his 1926 thesis "The Principles of Quantum Mechanics".
All quantum properties such as probability wave, commutation rules, uncertainty principle, and p = -i
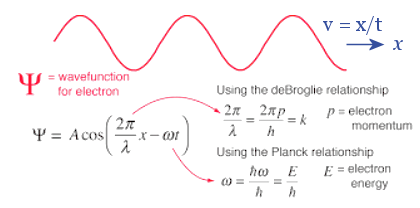
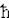 (d/dx) can be derived as shown in Figure 12-05a,d (with some hindsights ?). See "Short-cut to the Introduction of Quantum Theory" for more details, and the original book on "Quantum Mechanics" by L. Susskind, 2014.
(d/dx) can be derived as shown in Figure 12-05a,d (with some hindsights ?). See "Short-cut to the Introduction of Quantum Theory" for more details, and the original book on "Quantum Mechanics" by L. Susskind, 2014. =
=  ,
, 






 . Since the atomic nucleus move much slower than the electrons, the Born-Oppenheimer approximation can be adopted to separate the wave function
. Since the atomic nucleus move much slower than the electrons, the Born-Oppenheimer approximation can be adopted to separate the wave function