 |
 |
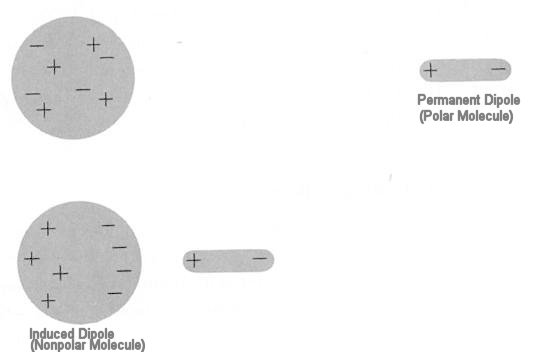
Such familiar aspects of the behavior of matter in bulk as friction, surface tension, viscosity, adhesion, cohesion, and so on also arise from van der Waals forces. The interaction is between dipole-dipole. It can be the interaction between a permanent and an induced dipole as shown in Figure 12-17 or between a time average dipole (due to fluctuations of charge) and an induced dipole as shown in Figure 12-18. The van der Waals interaction is about 10 times weaker than hydrogen bond. The stronger hydrogen bond can be considered as interaction between permanent dipoles.
|



