 |
organic compounds, carbon atoms are most likely to bond with hydrogen, oxygen, nitrogen, sulfur, and halogens such as chlorine. Hydrogen with one valence electron forms a single covalent bond. An octet is achieved by nitrogen forming three covalent bonds and so on. Figure 12-29 lists the covalent bonds for these elements in organic compounds. The solid line denotes a bond with two sharing electrons in between the atoms, while the dot represents the electron not in a bond. |
Figure 12-29 Covalent Bonds [view large image] |
 |
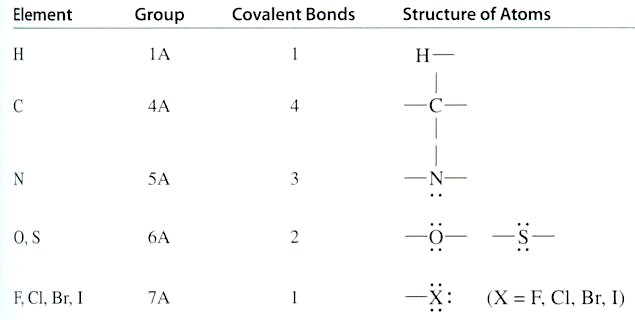
indicates that the four bonds are arranged as far apart as possible, and they have a tetrahedral shape as shown in diagram (a), Figure 12-30 for methane and ethane. Other three dimensional representations can be in the form of (b) the ball-and-stick model, (c) space-filling model, (d) wedges (toward the reader) and dashes (away from the reader). Diagram (e) depicts the structural formula, which shows how the atoms in a molecule are bonded together in two dimensions, (f) is the chemical formula in one dimension showing just the |
Figure 12-30 Organic Compounds [view large image] |
constituent atoms. Table 12-05 explains the naming convention for organic compounds. It serves to bring some order into the mind-boggling variety of organic compounds. |
| # of Carbon Atoms (n) | CnH2n+2 | CnH2n | CnHn | CnH2n+1OH | Examples |
|---|---|---|---|---|---|
| n = 1, meth- | -ane | -- | -yne | -anol | chloro-methane, meth-oxy-methane, methane-thiol |
| n = 2, eth- | -ane | -ene | -yne | -anol | eth-ane, eth-ene, eth-yne, eth-anol, ethan-amide |
| n = 3, prop- | -ane | -ene | -yne | -anol | prop-ane |
Table 12-05 Naming Convention for Organic Compounds
Note 1: The hydrogen atoms can be replaced by other atoms with corresponding number of bonds. For example, twohydrogen atoms with one bond each can be replaced by one oxygen atom carrying two bonds (ethanal).
But then the resulting compound would be categorized into another class and may not follow exactly the same
convention in the table (see Figure 12-32).
Note 2: Structural formula for the examples can be found in Figure 12-32.
 |
the shared electron closer to its nucleus) than carbon, with the exception of hydrogen (see Figure 12-31). A bond between identical atoms is nonpolar. A polar bond forms between atoms of different electronegativity values. However, when molecules consist of several polar bonds, the arrangement of the bonds determines whether it is a polar or nonpolar molecule. If a molecule contains a symmetrical arrangement of polar bonds so that the dipoles cancel out, the molecule is nonpolar. Figure 12-31 shows the polar and nonpolar configurations of some molecules. |
Figure 12-31 Polarity |
| Inorganic Compounds | Organic Compounds |
|---|---|
| A few compounds with carbon atom, e.g., CO2 | All organic compounds are carbon base |
| Elements joined by ionic or covalent bonds | Elements joined exclusively by covalent bonds |
| Most are ionic or polar covalent | Nonpolar, unless a more electronegative atom is present |
| Dissolve in water, may produce ions | Not soluble, unless a polar group is present or in organic liquids |
| High melting and boiling points | Low melting and boiling points |
| Vaporize at high temperature | Decompose by heat more easily |
| Flammability low | Flammability high |
| Reaction proceed quicker as solutions of the reactants are brought together | Reaction proceed at much slower rates in hours or days (except in living cell with enzymes) |
| Do not exhibit isomerism | May exist as isomers |
Table 12-06 Difference Between Inorganic and Organic Compounds
Note: Isomers are chemical compounds with identical chemical formula but different arrangements of elements. |
Table 12-05, Figure 12-32 shows the classification of organic compounds into fourteen functional groups. The hydrocarbons, which contain only carbon and hydrogen, are the simplest of the organic compounds. The alkanes (a functional group) contain only carbon-carbon single bonds. They are also called saturated hydrocarbons because they cannot add any more hydrogen atoms to the structure. The alkanes are not very reactive compared to compounds in other functional groups, but they serve as a basic structure for the rest of the organic molecules. Hydrocarbons with double or triple bonds are called unsaturated hydrocarbons, because they can add atoms of hydrogen, oxygen, or a halogen. The alkenes contain a functional group that is a double bond between two adjacent carbon atoms; one of these bond can be linked to other atoms. Alkynes contain a triple bond. The alkenes and the alkynes are much more reactive than the single-bonded alkanes. Usually the addition of other molecules such as oxygen, sulfur, |
Figure 12-32 Functional Groups [view large image] |
nitrogen, phosphorus or halogen would make the compound more reactive. The structural formula for all the functional groups is shown in Figure 12-32. |
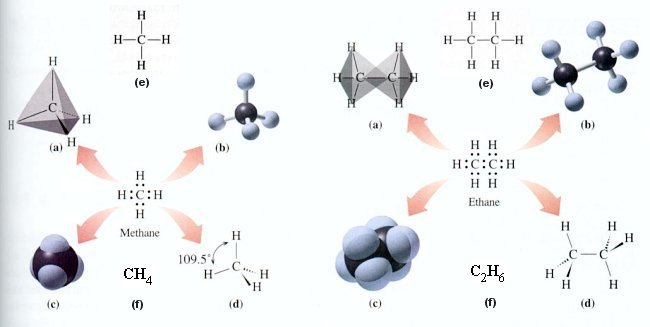
- Plastics - Polymers are organic compounds which contain enormously long chains of atoms. These chains are made up of small repeating units of molecules called monomers. Some polymers, such as plastics are a large group of synthetic polymers which have had a tremendous impact on our everyday lives. All plastics have a number of useful qualities in common. They are strong as well as being light and flexible, easily colored and molded into shape. In addition, they are good heat insulators and are rot and corrosion-proof. The alkene,
and ethene form the basis of many important plastics. For example, under the right conditions, ethene molecules will react with each other, opening up their double bonds and joining together to form the polymer (poly)ethene, or polythene (Figure 12-33). Most plastics are made from raw materials derived from crude oil. They are difficult to recycle, most plastics cannot be burned as they release toxic fumes. The non-biodegradable rubbish is buried in huge holes dug deep into the ground called landfill sites. The plastics in there will give off methane gas, and form toxic slime leaking into underground water supplies. Figure 12-33 Plastics
[view large image]Figure 12-34 Foods

- Fuels - Hydrocarbons, particularly alkanes, are the compounds that make up natural gas, motor oils, gasoline, and other fuels. The coal and crude oil that provides these hydrocarbons was formed 200 million years ago from the pressures on buried organic matter deep in the earth. Because the hydrocarbons are flammable we use compounds such as methane (CH4), propane (C3H8), and butane (C4H10) as energy sources.
- Alcohol - Ethyl alcohol (C2H5OH) is the alcohol found in alcoholic beverage. Isopropyl alcohol (C3H7OH) is another alcohol commonly used to disinfect skin before giving injections and to treat cuts.
- Solvent - Acetone is used as an organic solvent because it dissolves a wide variety of organic substances.
- Food flavorings - Ketones and aldehydes are used in food industry to produce artificial flavorings such as vanilla, cinnamon, and spearmint. These substances are usually dissolved in alcohol because they are not very soluble in water. The aldehyde butyraldehyde (C4H8O) adds a "buttery" taste to foods and margarine.
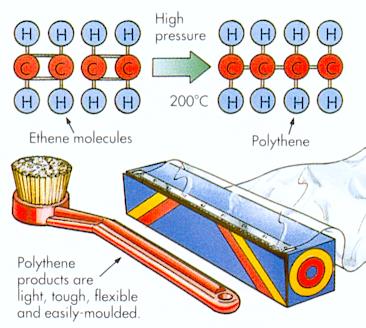
- Vinegar and juices - The sour tastes of vinegar and fruit juices and the pain from ant stings are all due to carboxylic acids. Acetic acid is the carboxylic acid that makes up vinegar. Aspirin also contains a carboxylic acid group. Esters found in fruits produces the pleasant aromas and tastes of bananas, oranges, pears, and pineapples (Figure 12-34). Esters are also used as solvents in many household cleaners, polishes, and glues.
- Fish - One of the characteristics of fish is their odor, which is due to amines. Amines are produced when proteins decay, they give off a particularly pungent and offensive odor (Figure 12-34).
- Dried foods - Since carbohydrates and proteins in death organisms decay via hydrolysis. Foods can be preserved by removing the water within. This method was used extensively in the past when preservative and refrigeration were not available.
- Stimulants - Alkaloids are biologically active amines synthesized by plants to ward off insects and animals. Some typical alkaloids include caffeine, nicotine, and histamine. Many are painkillers and hallucinogens such as morphine, LSD, marijuana, and cocaine. Certain parts of our neurons have receptor sites that respond to the various alkaloids. By modifying the structures of certain alkaloids to eliminate side effects, chemists have synthesized painkillers and drugs such as Novocain, Codeine, and Valium.
- Carbohydrates - In plants, photosynthesis combines CO2 and water to form monosaccharides such as glucose and polysaccharides such as starch and cellulose. Glucose has a six-carbon chain with an aldehyde group and several hydroxyl groups. When glucose molecules join by ether links, long polymers of complex carbohydrates call polysaccharides are formed, which are known as starch and cellulose. A difference in the direction of the bond between the glucose molecules allows us to digest the starches in our cereals and bread, but not the cellulose in their packaging.
- Lipids - Lipids containing carbon, hydrogen, and oxygen make up a variety of components in our body such as body fat. Body fat is composed of fatty acids, which are carboxylic acids with long carbon chains, bonded to glycerol by ester bonds. Because most lipids are insoluble, they play a major role in the structure of cell walls, which separate cell contents from aqueous fluids outside the cells.
- Protein - Proteins are composed of carbon, hydrogen, oxygen, and nitrogen. These large polymers consist of small molecules called amino acids that have both amine and carboxylic acid functional groups. The order of amino acids in a protein determines the structure and function of the protein in the body. There are proteins such hemoglobin that transport oxygen to the cells, contract our muscles, form antibodies, make up hair, skin, and enzymes that catalyze biological reactions.
- Nucleic acids - Nucleic acids are long chains of nucleotides, which contain a carbohydrate called ribose, a nitrogen base, and a phosphate group. The nucleic acids carry the messages for the production of all the proteins in the cells. It contains the genetic material for the reproduction of next generation.