
| Home Page | Overview | Site Map | Index | Appendix | Illustration | About | Contact | Update | FAQ |
 |
 |
atoms, the internal energy of a solid is assumed to reside in elastic standing waves. These waves, like electromagnetic waves in a cavity, have quantized energy contents. A quantum of vibrational energy in a solid is called a "phonon", and it travels with the speed of sound. The concept of phonones is quite general and has applications in connection with the thermal conductivity of some solids, the electrical conductivity in crystals, and in superconductivity. Calculation also reveals that contribution of electron |
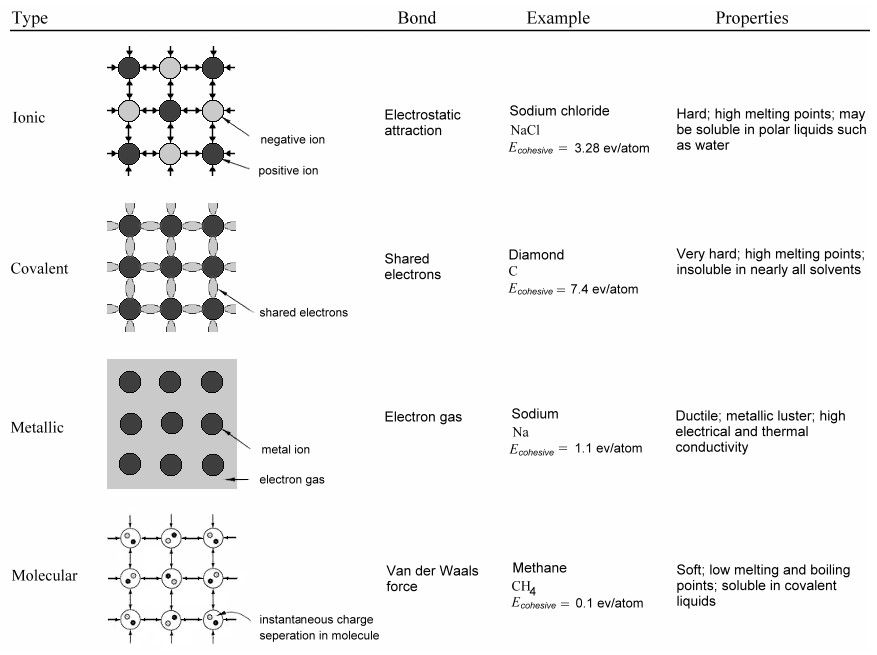
Figure 13-05a1 Crystal Types [view large image] |
Figure 13-05a2 Specific Heats |
gas to the heat capacity is negligible except at very low temperature. The various models are shown graphically in Figure 13-05b1. |
 Q to raise the temperature
Q to raise the temperature  T by 1 degree K (or C), i.e., c =
T by 1 degree K (or C), i.e., c =  Q/
Q/ T. Specific heat capacity (or just specific heat) is the amount to increase 1o in one unit mass (e.g. 1 gram). The molar heat is even more precise by referring to 1 mole
T. Specific heat capacity (or just specific heat) is the amount to increase 1o in one unit mass (e.g. 1 gram). The molar heat is even more precise by referring to 1 mole
 |
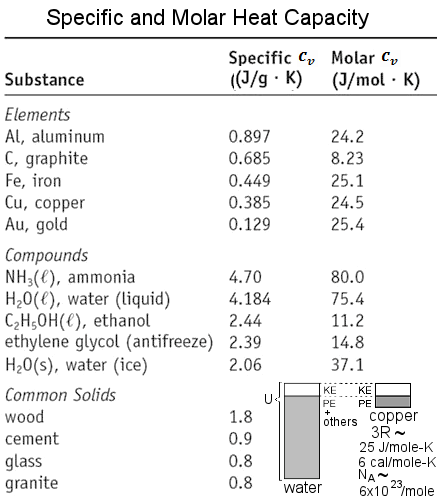 |
(~ 6x1023 particles in 1 atomic weight of the substance / mole = NA = Avogadro's number). Figure 13-05b2 lists the specific and molar heat for some selected substances. The conversion from J/g-K to J/mole-K is by multiplying the atomic weight. It leads to some curious situations that the relative amount is reversed after the conversion, (e.g.. graphite has higher value of specific heat than gold, but it is the opposed in molar heat because the latter has higher atomic weight). Since the hot or warm sensation is related to the temperature sensors on our skin, thus we would feel cooler for substance with high heat capacity from same amount of heat input (see insert in Figure 13-05a2). |
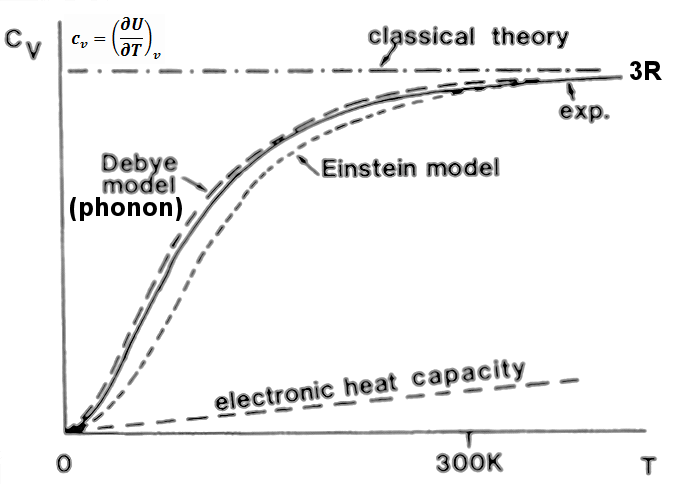
Figure 13-05b1 Specific Heat Theories [view large image] |
Figure 13-05b2 List of Specific Heat | The 4 different models (as shown in Figure 13-05b1) that try to explain the behavior of heat capacity are derived in the followings : |

 , where
, where  = (K/m)1/2/ 2
= (K/m)1/2/ 2 . Using the same Maxwell-Boltzmann energy distribution function, the averaged energy becomes :
. Using the same Maxwell-Boltzmann energy distribution function, the averaged energy becomes :
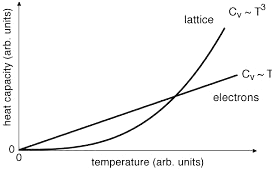 |
(3) Electron Gas - The electron gas contribution to heat capacity can be estimated by considering that only those electrons near the Fermi energy EF can absorb the heat energy kT, i.e., N ~ NA(kT/EF). Therefore, the internal energy U ~ Nx(3/2)kT giving cv ~ (3/2)(kT/EF)R, which is very small comparing to the classical estimate of 3R and is not entirely satisfy with the T3 temperature dependence at low temperature (Figures 13-05b1 and 05b3) |
Figure 13-05b3 Electron Gas Specific Heat |
 |

|
Figure 13-05b4 Phonon |
See Figure 13-05b4 to compare. |