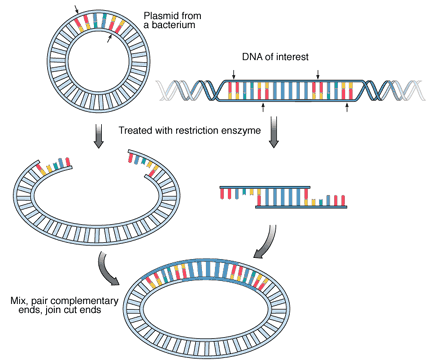
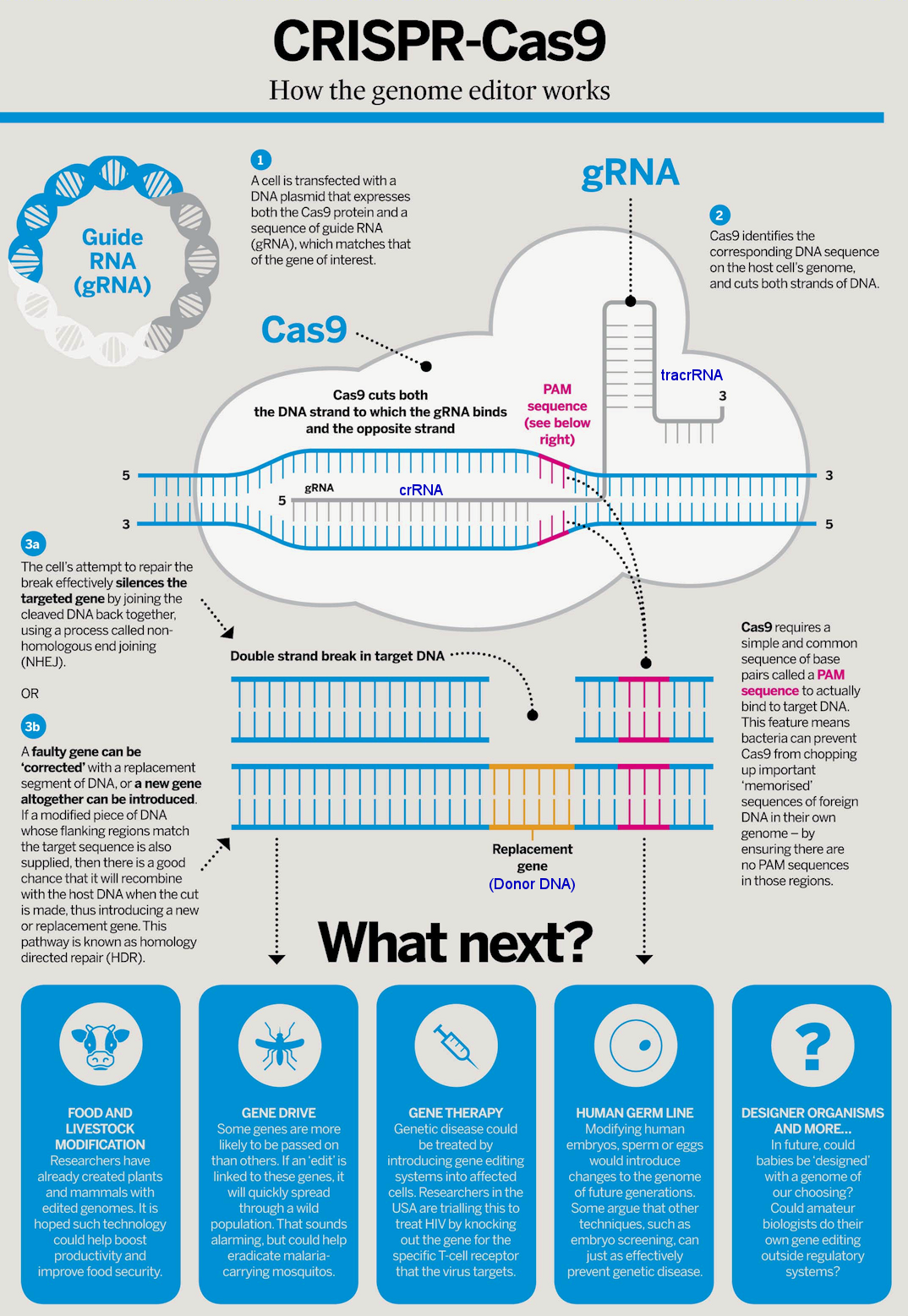
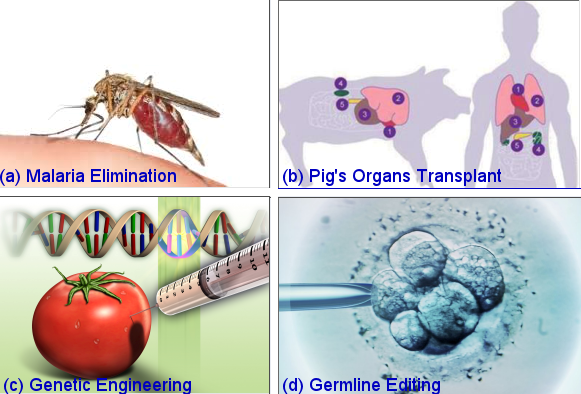
 |
Actually up to the summer of 2016, CRISPR-Cas9 editing is used mostly to delete genes that cause diseases. Gene insertion is more difficult with much lower efficiency. The existing system has many problems including : the CRISPR-Cas9 package is too large to load into virus for delivery, and it depends on the detection of PAM sequence to cut. Researches are in progress to use mini-Cas9, and Cpf1 as alternatives to address the shortcomings. It is found that disabling Cas9 could alter the genetic code one base at a time, but would not cut. Then there is the controversial NgAgo protein, which slices DNA at a pre-determined site without a gRNA or a PAM sequence. Laboratories all over the world have so far failed to reproduce the result (the authors retract the claim from Nature on August, 2017).
|