 |
The first thermochemistry law (proposed in 1780) states that: the quantity of heat required to decompose a compound into its elements is equal to the heat evolved when that compound is formed from its elements. The second law of thermochemistry (also known as Hess law) states that the heat change in a chemical reaction is the same whether it takes place in one or |
Figure 12-19 Hess Law |
several stages. That is, the heat change depends on the initial and final states only (Figure 12-19). |
 |
|
Figure 12-20 Reaction Rate [view large image] |
way to speed up a reaction is to lower the energy of activation. This can be done by adding a catalyst, which speeds up the reaction but is not itself changed or used up. |
 |
 |
are not important. When solids or liquids form solutions, there must be an attraction between the solute particles and the solvent particles. Otherwise, the particles do not mix and no solution forms. Compounds containing nonpolar molecules such as iodine, oil, or grease do not dissolve in water because water is polar. The polarities of a solute and a solvent must be similar in order to form a solution. It is found as early as the 1800s that whenever a substance is dissolved in a liquid the vapor pressure (the pressure at which the rate of evaporation is equal to the rate of condensation) |
Figure 12-21 Solution |
Figure 12-22 Types of Solution [view large image] |
of the latter is lowered. Such behaviour has the effect of rising the boiling point and lowering the freezing point of the solution. It also produces |
 |
 |
There are two main groups of electrolytic conductors; the first consists of pure substances, e.g., fused salts, and the second of solutions. The most thoroughly studied examples of the latter are solutions of acids, bases and salts in water. Sodium chloride (NaCl) is a strong electrolyte, which dissociates in water into hydrated ions of Na+(aq) and Cl-(aq). As shown in Figure 12-23a, the light bulb glows as these ions provide a path for current flow in a circuit of : light bulb - battery - electrolytic solution - light bulb. HF is a weak electrolyte only generates a dim light. While nonelectrolytic substances such as sugar block the passage of electricity altogether. Figure 12-23b shows the essential parts of a |
Figure 12-23a Electro- lyte [view large image] |
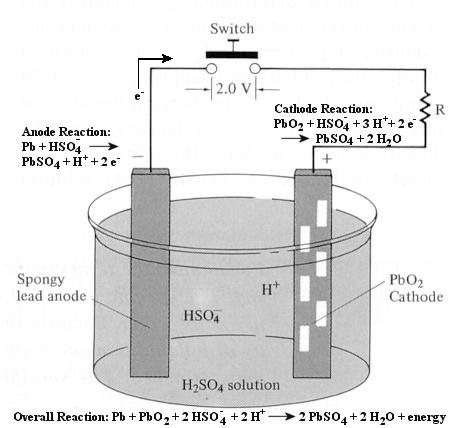
Figure 12-23b Battery |
lead storage battery. When the switch in the battery circuit is closed, reactions take place at the anode and the cathode as shown in the diagram. The battery |
 |
fusion. The temperature remains constant during the change of phase. Once the conversion is complete, the temperature of the water starts to rise again from c to d with a different rate since the specific heat (capacity to absorb heat) of water is greater than that of ice. Similar phase change occurs from d to e for water changing into steam. It takes longer time for the phase change from d to e, because the heat of vaporization (~ 540 cal/gm) is about 7 times higher than the heat of fusion. If the heating process continues from e to f, the gas would be called "superheated steam". The process reversed when heat is removed from the container. |
Figure 12-24 Phase Change |
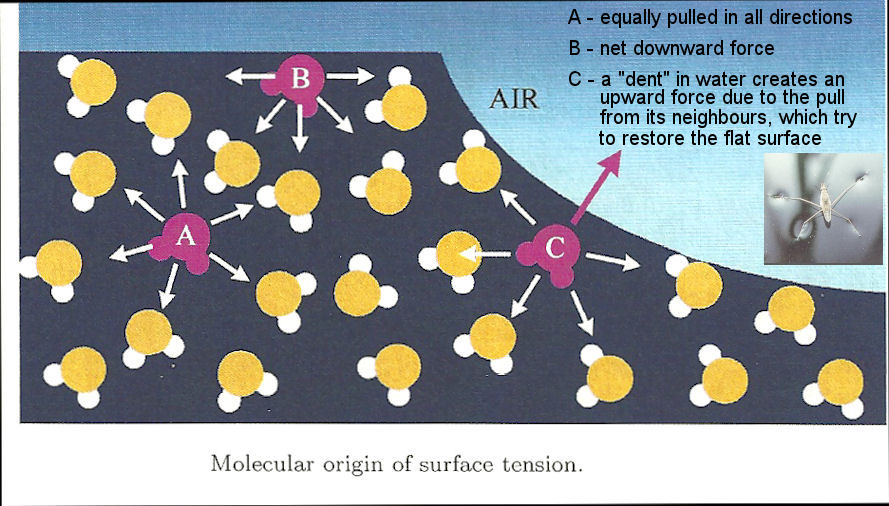 |
the smallest possible area; it is for this reason that drops of liquid and bubbles of gas in a liquid become spherical, a needle floats on the top of water, certain water bugs can travel across the surface of a pond or lake (see Figure 12-25a). When compounds called surfactants (Figure 12-05b) are added to water, they disrupt the hydrogen bonding between the water molecules. As a result, the surface tensions decreased and the water spreads out rather than forming drops or curved surface (Figure 12-25c). Soap, detergents, shampoos, and fabric softeners, are example of surfactants we use every day. |
Figure 12-25a Surface Tension [view large image] |
 |
 |
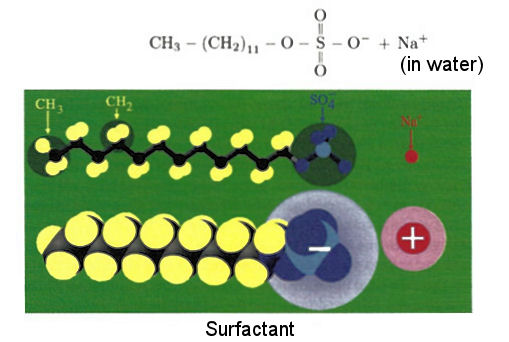
Surfactant is a class of organic chemicals call amphiphiles (amphi-, meaning both), which has a hydrophilic (water loving) head and a hydrophobic (water hating) tail. An example of sodium dodecyl sulfate (SDS) molecule is shown in Figure 12-25b. When these molecules are dissolved in water the tails manage to avoid the water molecules by either forming a thin layer on the surface with the tail sticking out or wrapping into a sphere with the head facing the water molecules (Figure 12-25c). Oils and fats, which dislike |
Figure 12-25b Surfactant |
Figure 12-25c Surfactant in Water [view large image] |
contact with water, are easily incorporated into the sphere and washed away in our daily cleaning actions. |
