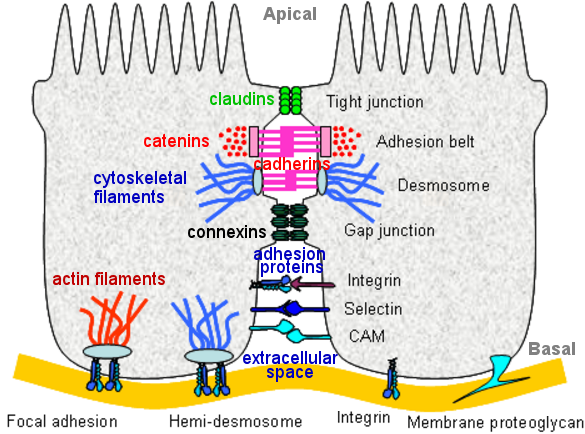
| Name |
Component(s) |
Function(s) |
| CAM |
Transmembrane receptor |
Umbrella designation for "Cell Adhesion Molecule" |
| Integrin |
Surface receptor (a protein) |
Responsible for C-C and C-ECM monitoring and communication |
| Selectin |
Transmembrane polypeptide |
Mediating migration of circulatory cells |
| Gap Junction |
Connexin + Channel |
Providing direct contact between the cytoplasm of two cells |
| Tight Junction |
Claudin + Occludin + Actin filaments + ... etc |
Creating very close contact between the membrane of two cells |
| Adhesion Belt |
Cadherin + Catenins + Actin filaments + ... etc |
Regulating cell shape, maintain tissue integrity, etc |
| Desmosome |
Cadherin + Desmoplakin + Cytoskeletal filaments |
Providing strong adhesion between cells |
| Hemi-desmosome |
~ Desmosome with Cadherin replaced by Integrin |
Promoting adhesion of epithelial cell to the basement membrane. |
| Focal Adhesion |
Integrin + Talin + Actin filaments |
Providing mechanical links between actin filaments and ECM |
| Proteoglycan |
Polysaccharide chains |
Regulating the movement of molecules in ECM, and signaling |
 |
Chemical Bond - Atoms can bind together to form molecules by the attraction of opposite electric charges. Depending on the electronic configurations of the atoms, the bonds are classified (in order of the attractive strength) as ionic, covalent, metallic, hydrogen, and the weakest dipole-dipole bond via van der Waals force (Figure 12-25g). Usually, individual molecules are invisible with unaided eyes (too small in size). It is through the cycle of condensation, melting, dissolving (in liquid), and precipitation that the individual atoms and molecules come together to form small crystals of a few centimeter in size (enough to be seen). The elements are naturally scattered all over, they come together to form rocks and minerals by condensation or precipitation at different temperature, but they do not cleanly segregate one type from the others. That's why it is so valuable to have mineral of relatively pure compound weighed a few carat (called gemstone, 1 carat = 0.2 gm) shown below :

|
|
See more from "Rocks, Minerals, and Gemstones" about this kind of adhesion on inanimate objects in nature. |

 formed by the tangential at the edge. It is inversely proportional to wetting (see formulas in Figure 12-25d).
formed by the tangential at the edge. It is inversely proportional to wetting (see formulas in Figure 12-25d).